
Tom Goddard
February 11, 2026
ChimeraX can run the OpenFold 3 structure prediction method to compute atomic structures of proteins and nucleic acids, including modified residues, ligands and ions on your laptop or desktop computer. A ChimeraX graphical user interface (menu Tools / Structure Prediction / OpenFold) and ChimeraX command (openfold) are provided to make predictions. ChimeraX daily builds dated February 13, 2026 and newer use OpenFold 3 preview. OpenFold 3 is fully open source under the Apache 2.0 license.
When you first start the OpenFold tool within ChimeraX (menu Tools / Structure Prediction / OpenFold) it will show a button Install OpenFold. OpenFold is a large software package, taking about 1 Gbyte of disk space and uses the Torch machine learning package. It also requires the neural network weights (2 Gbytes) to make predictions. Downloading and installing can take 10 minutes or more depending on network speed OpenFold will be installed in your home directory in ~/openfold3 and the network weights and ~/.openfold3.
The ChimeraX openfold install command can also be used to do this one time installation.

|
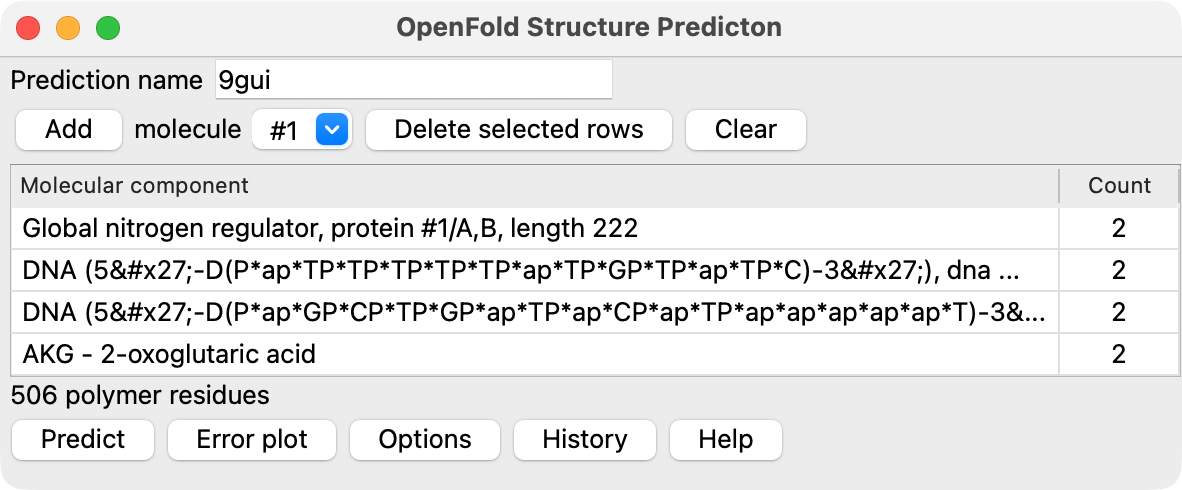
Menu of molecular components
|
To predict a structure made up of proteins, nucleic acids and small molecules you first specify all the molecular components. Choose entries from the Add menu and press the Add button to add them to your assembly specification in the table below. You can specify component molecules in several ways.
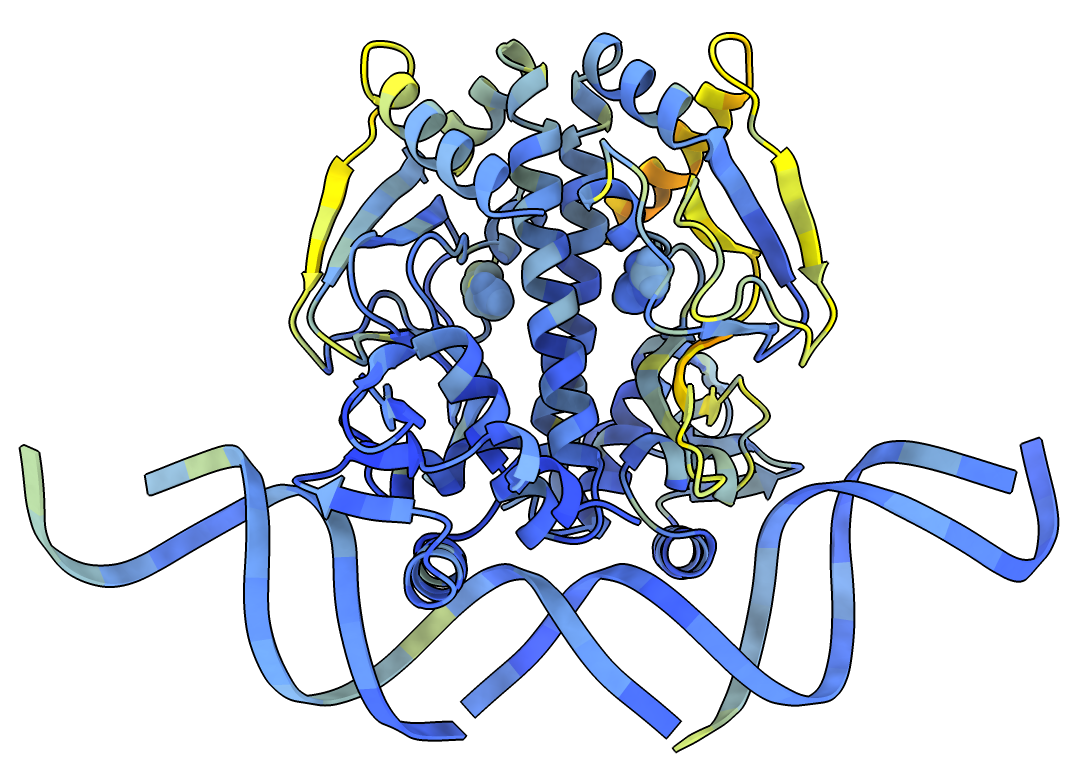
NTCA transcription factor bound to DNA |
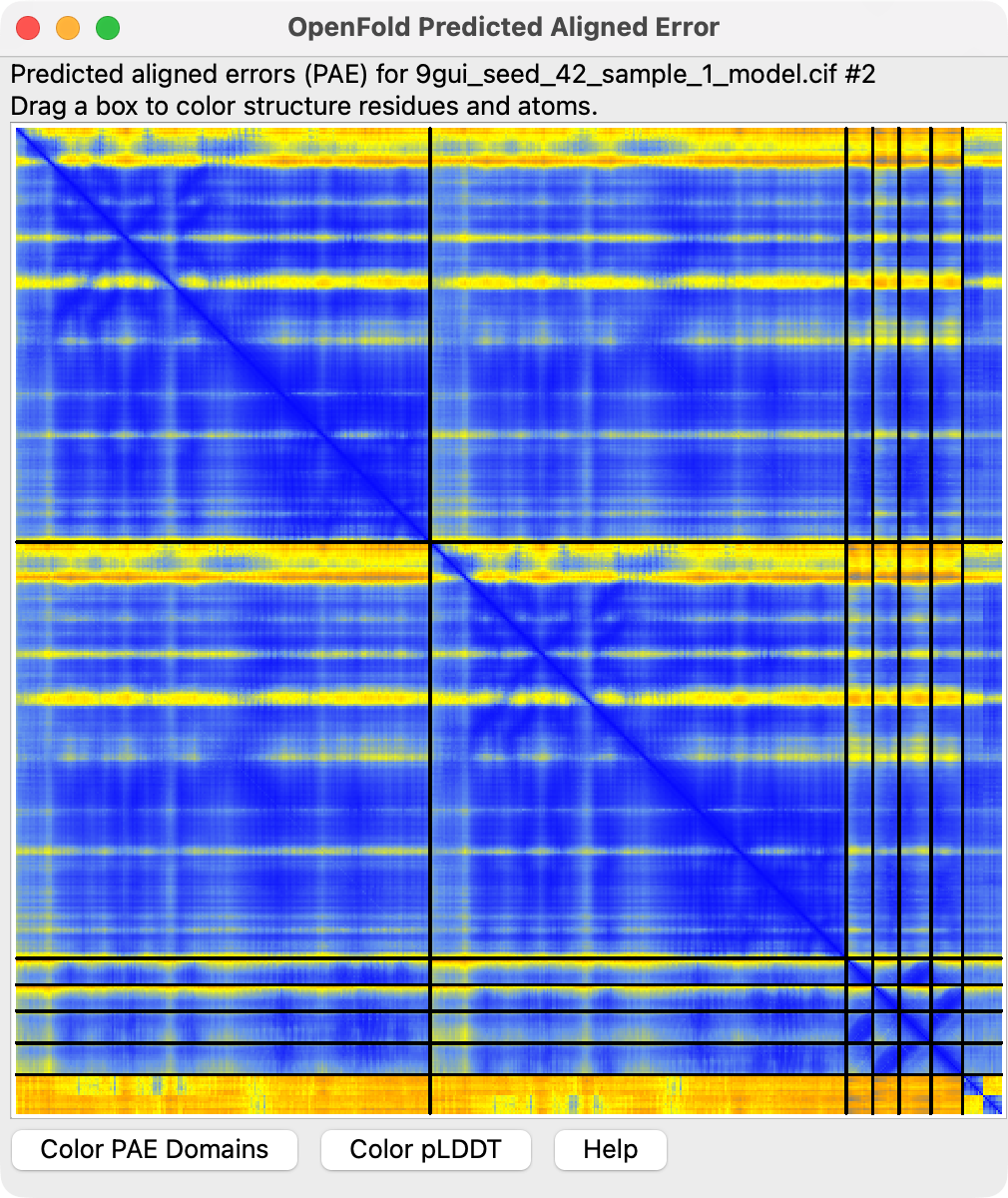
Predicted aligned error |
Components can be added multiple times to have more instances of that molecule in the assembly. Press the Predict button after the assembly is completed by adding each component to start the prediction. A Stop button will be shown while the prediction runs to terminate the prediction, discarding the partial computation so you can start another prediction.
The results are put on your desktop in a new folder openfold/<prediction-name> where the prediction name can be specified at the top of the ChimeraX OpenFold panel. Using the Options described below you can change where the result files are placed.
Predictions for small assemblies, for example 500 residues and ligand atoms, take one to several minutes depending on the computer (e.g. Nvidia GPU vs CPU only). Predictions run in the background (a separate process) so you can continue to use ChimeraX while the calculation runs. The predicted structure will be opened in ChimeraX when the calculation completes. If the assembly specification involved proteins or nucleic acids specified using chains of open models, the predicted structure will be aligned (using matchmaker) to the open model for the first such component.
The prediction will be colored using the standard AlphaFold pLDDT type of coloring where blue indicates high confidence, yellow and red moderate to low confidence.
Per-residue-pair estimates of prediction confidence can be displayed by pressing the Error Plot button to show the predicted aligned error.
OpenFold structure predictions use a lot of memory and compute resources on your computer that limits the size of the structure that can be predicted. See the run times section below for example run times and size limits.
Mac. It works well on Mac M-series (M1,M2,M3,M4) laptop and desktop computers predicting small 100 residue structures in 1 minute and up to 1200 residues in about 15 minutes with 32 GB of memory. With 16 GB of memory it can only predict about 350 amino acids taking about 5 minutes, with larger predictions running out of memory.
Nvidia GPUs on Windows and Linux. Nvidia GPUs on Windows and Linux computers also provide good performance. On Linux with an Nvidia GPU with 24 GB of graphics memory (e.g. Nvidia RTX 3090 or 4090) it can predict about 1300 residues in about 4 minutes, larger sizes run out of memory. Testing on Windows with less GPU memory, 12 GB (e.g. Nvidia RTX 4070) predicts up to 1000 residues. On Windows with 8 GB of GPU memory (e.g Nvidia RTX 3070) predictions of about 700 residues. Large predictions on Nvidia GPUs on Linux run out of memory and fail. On Windows the prediction will fallback to using CPU memory allowing larger structure predictions but taking immensely longer run times (10-30 times longer).
Intel CPU. Predictions only utilizing an Intel CPU are very slow, for example 1.5 hours for 900 residues. Expected size limits are about 350 residues with 16 GB, 1000 residues with 32 GB, and about 1600 residues with 64 GB. Run time is expected to increase as the square of the number of residues.
Here are run times for a few desktop and laptop computers for predicting various size molecular assemblies from the Protein Databank using the ChimeraX OpenFold 3 fork from February 11, 2026.
OpenFold prediction times in minutes, using cached MSAs. Tokens is the number of polymer residues plus ligand atoms.
| PDB code | Tokens | Mac M1 16 GB | Mac M1 Max 32 GB | Mac M2 Ultra 64 GB | Linux i9 CPU 64 GB | Linux Nvidia 4090 | Windows i7 CPU 64 GB | Windows Nvidia 3070 | Number of residues and atoms and prediction error |
|---|---|---|---|---|---|---|---|---|---|
| 8rf4 | 129 | 1.5 | 1.0 | 0.8 | 1.4 | 0.7 | 1.5 | 118 amino acids, 11 ligand atoms, 1.5A RMSD 118 residues | |
| 9gui | 526 | 18 | 4.3 | 2.5 | 16 | 1.0 | Protein dimer, DNA, 2 ligands, 1.4A RMSD for protein | ||
| 9moj | 660 | 30 | 6.6 | 3.7 | 25 | 1.1 | 30 | 3.3 | 660 amino acids, heterotetramer, 0.9A RMSD 125 residues |
| 9h1k | 671 | 6.9 | 4.0 | 1.1 | 560 amino acids, 59 rna bases, 52 ligand atoms,
1.2A RMSD for 261 residues, RNA wrong | ||||
| 9b3h | 911 | 38 | 7.6 | 1.5 | 911 amino acids, heterodimer, 1A RMSD 503 residues | ||||
| 9fz5 | 1025 | 11 | 1.7 | 1025 amino acids, heterotrimer, 2.2A RMSD 740 residues | |||||
| 9mcw | 1154 | 16 | 2.3 | 1154 rna bases, homodimer, wrong dimer and monomer conformations | |||||
| 8sa0 | 1371 | 30 | 2.6 | 1274 amino acids, 97 ligand atoms, 2.1A RMSD 1151 residues | |||||
| 9gh4 | 1467 | 2.9 | Protein homotrimer, monomer 489 residues, 1.5A RMSD for 250 residues | ||||||
| 9enr | 1794 | 4.1 | Protein monomer, 4 ligands, 2.9A RMSD for 1551 residues | ||||||
| 1dpp | 2028 | 5.2 | Homotetramer, 0.47A RMSD for 507 residues | ||||||
| 9hma | 2218 | failed | Protein monomer |
Mac GPU acceleration. The reported Mac performance is for Mac M1/M2/M3/M4 series GPUs. OpenFold uses machine learning package torch which has GPU acceleration called Metal Performance Shaders (MPS) on these Mac M series GPUs which have speed up to 2-5x slower than an Nvidia 4090 but with the advantage that the Mac can handle larger molecular systems using the unified computer memory (e.g. 32 or 64 GB). With 16 GB prediction size is limited to 350 residues. Older Mac Intel machines do not have GPU acceleration in Torch and run at speeds similar to Windows Intel CPU-only predictions.
Windows Nvidia GPU performance. The above table shows a significant slow-down in predictions beyond about 600 residues on Windows with Nvidia 3070 (8 GB) and 4070 (12 GB) graphics. This is probably because the GPU memory is insufficient for larger structures and the machine learning toolkit falls back to a mix of CPU and GPU calculation. Notice that the 4070 GPU took more time than the 3070 GPU for large structures probably because the CPU on the 4070 machine (i5-6700K) is significantly slower than the CPU on the 3070 machine (i7-12900K).
Linux Nvidia GPU out of memory. On Linux Nvidia 4090 with 24 GB of GPU memory the maximum prediction appears to be about 2000 residues plus ligand atoms before an "out of memory" error occurs. This contrasts with Windows where Torch appears to fallback to using CPU and not run out of GPU memory.
CUDA use of bfloat16. On Nvidia CUDA the ChimeraX OpenFold uses 16-bit floating point (bfloat16, bf16-mixed torch lightning) which allows larger predictions then on non-CUDA systems where 32-bit float is used. Torch only supports hardware accelerate bfloat16 CUDA .
Several predictions can be run each with a different ligand to see how it binds to the rest of the molecular assembly. Use the Add molecule "each ligand SMILES string" menu entry. Paste in a set of SMILES strings with ligand names. Each line should have a ligand name, comma, followed by a SMILES string. The ligand names will be used as the predicted structure file names. Alternatively you can just enter one SMILES string per line without a name and the ligands will be named "ligand1", "ligand2", .... Press the Add button after pasting in the set of ligands, then the Predict button to run the series of predictions.
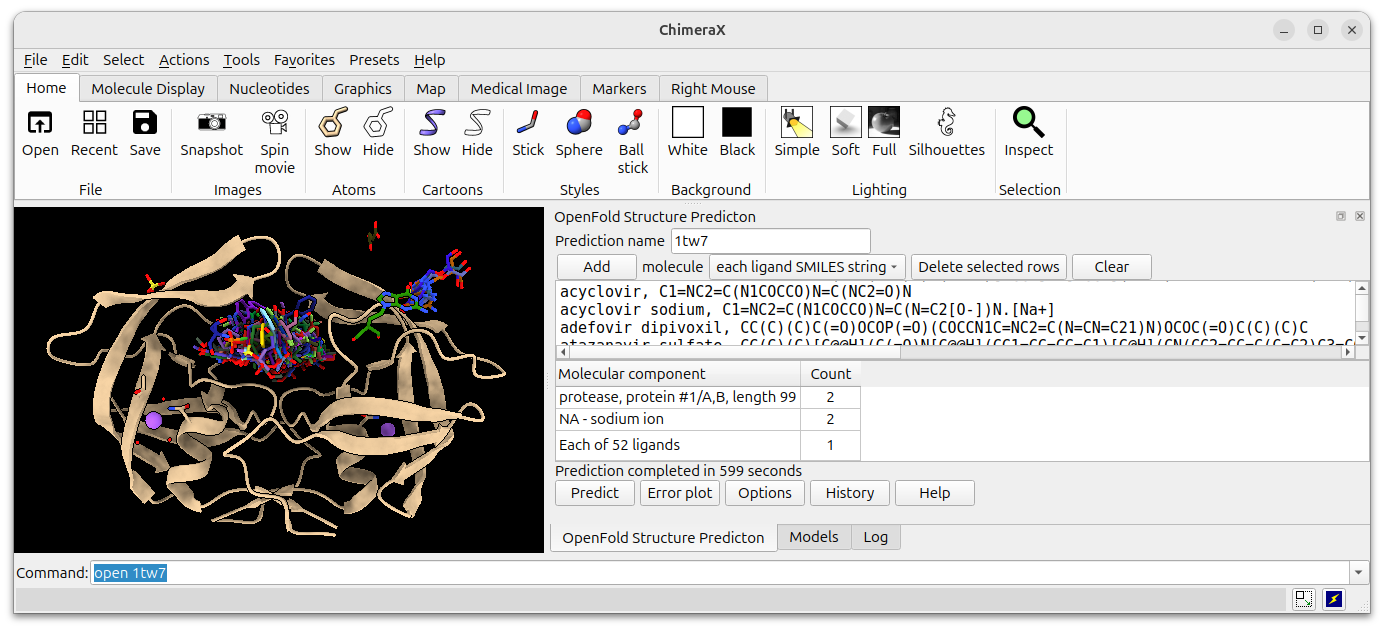
Example input for 52 anti-viral ligands run against HIV protease. Predictions completed in 10 minutes on an Nvidia 4090 GPU. | 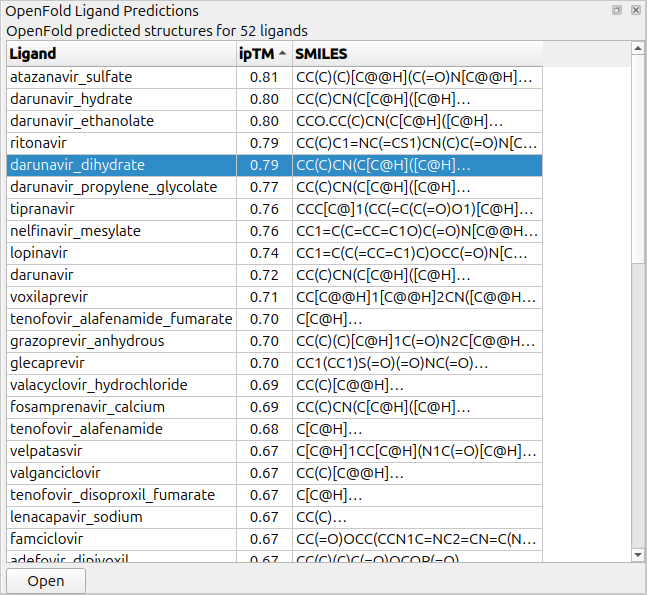
Table of prediction ipTM confidence for ligands. |
Progress messages will appear in the OpenFold panel as the structures are predicted for each ligand. The ipTM scores is a crude prediction of binding confidence. When the predictions complete a table of results giving ligand name, ipTM and SMILES string will appear. The table can be sorted by clicking on the column headers. Selecting rows of the table and pressing the Open button will open the selected structure predictions. The table is written as comma-separated values to the directory where OpenFold was run, for example hiv_protease/hiv_protease.oflig in the case shown. This file will appear in the file history thumbnails when you start ChimeraX in future sessions and clicking on the thumbnail will reshow the table.
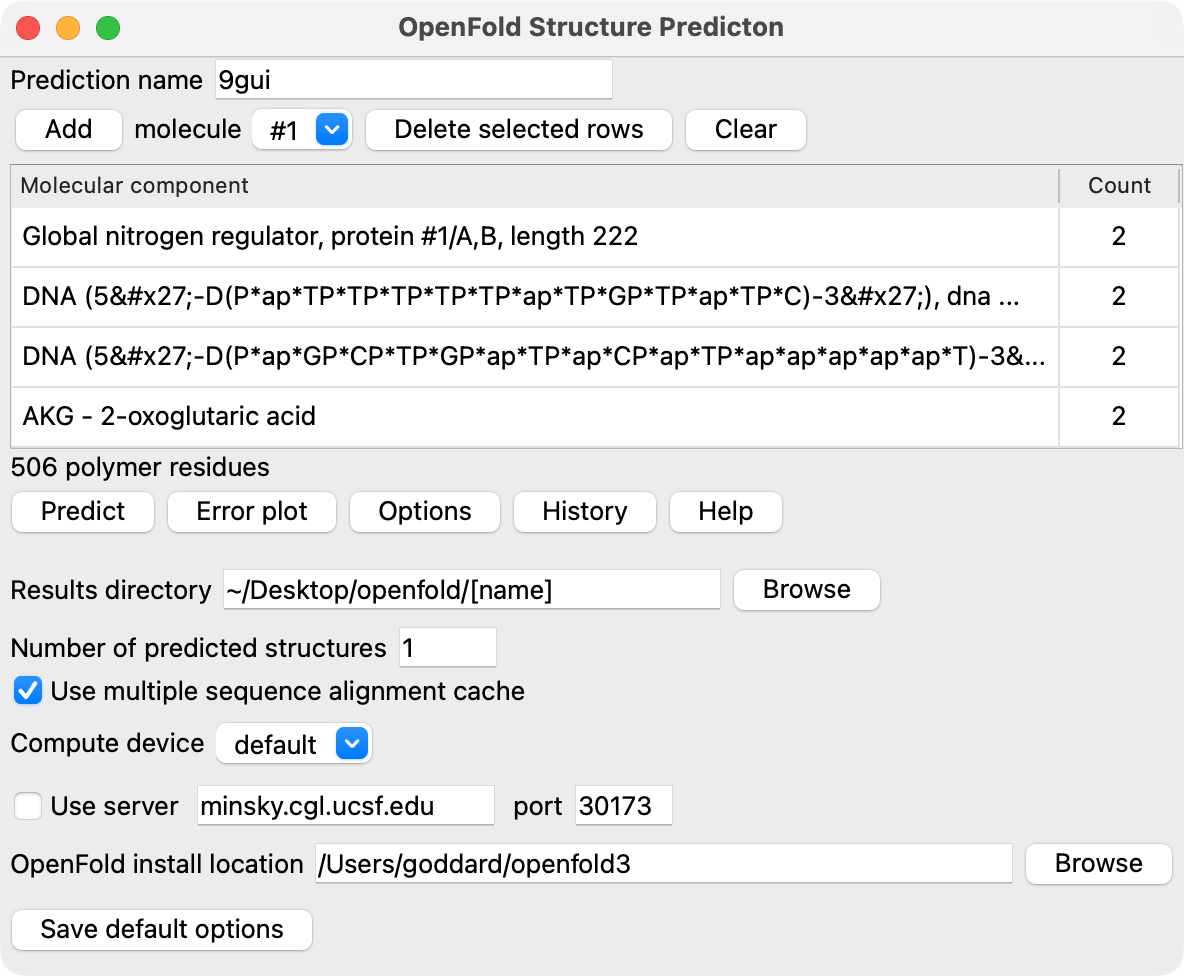
Pressing the Options button shows additional settings for openfold predictions.
Additional advanced options are available by using the ChimeraX openfold command.
|
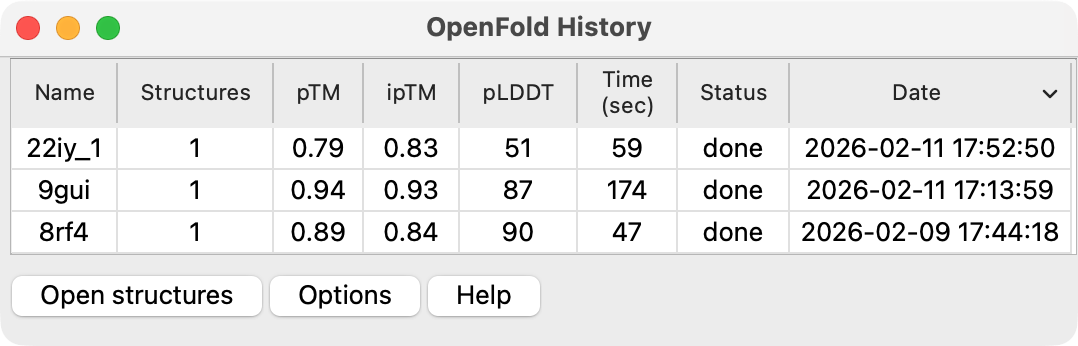
ChimeraX menu entry Tools / Structure Prediction / OpenFold History lists a table of past OpenFold predictions. Selecting rows of the table and pressing the Open structures button will open those structures. The table is filled by scanning the OpenFold prediction directories located in the directory where ChimeraX puts new OpenFold predictions (by default ~/Desktop/openfold). If a prediction computed multiple structures they will all be aligned to the first structure of that prediction when opened (using the ChimeraX matchmaker command). The directory containing the prediction directories and the choice to align can be specified in the panel shown by pressing the Options button.
Directories are identified as containing OpenFold predictions if they contain a file named "command". When ChimeraX runs a prediction it saves the openfold command including all its arguments are listed in this file.
Fetching server predictionsIf there are server predictions that did not complete before quitting ChimeraX, then when ChimeraX is run again the OpenFold History panel will show a button Fetch from server next to the Open structures button. This button fetches any server predictions that have completed and opens them. Predictions that have not yet completed are noted in the Log after pressing this button.
The ChimeraX OpenFold graphical interface runs a prediction by running the ChimeraX openfold command. That command is recorded in the ChimeraX Log panel, and looking at that command can help you understand the command options.
openfold predict [sequences] [protein sequences] [dna sequences] [rna sequences]
[ligands residue-spec] [excludeLigands ccd-codes]
[ligandCcd ccd-codes] [ligandSmiles smiles-string]
[forEachSmilesLigand name,smiles-string,name,smiles-string...]
[name prediction-name] [resultsDirectory directory] [samples n] [seed n]
[device default|cpu|gpu] [precision 32-true | bf16-mixed | 16-true | bf16-true]
[useServer true | false] [serverHost hostname] [serverPort port]
[useMsaCache true|false] [msaOnly true|false]
[open true|false] [installLocation directory] [wait true|false]
Options descriptions
openfold install [directory] [downloadModelWeights true | false] branch name
The openfold install command creates a Python virtual environment to install the ChimeraX OpenFold fork Github. If no directory is specified then ~/openfold3 in the user's home directory is used. The directory will be created or if it already exists must be empty. It then downloads the OpenFold network parameters and Chemical Component Dictionary to ~/.openfold3. Finally it makes an index of the atom counts for each CCD code so that the ChimeraX OpenFold interface can report the total number of tokens (residues plus ligand atoms) in an assembly in order to judge whether the computer has enough memory to make the requested prediction.
The install uses a fork of the OpenFold repository https://github.com/RBVI/openfold-3. It uses git branch chimerax of this fork unless the branch option is specified in which case it installs the specified branch. The branch option is for testing new versions of OpenFold. The default branch is chimerax_openfold.
The install process executes these commands to make the virtual environment and install OpenFold. It uses the ChimeraX Python executable to create the virtual environment. OpenFold will no longer work if ChimeraX is moved or deleted and will need to be reinstalled in that case. It will also no longer work if the openfold directory itself is moved since the openfold executable refers to the install location to find python.
The ChimeraX openfold install command creates a Python virtual environment and installs openfold and downloads the openfold weights. On Windows it installs a version of torch with CUDA 12.6 support before installing openfold if Nvidia graphics is detected.
python -m venv directory
directory/bin/python -m pip install torch --index-url https://download.pytorch.org/whl/cu126 # On Windows with Nvidia GPU only.
directory/bin/python -m pip install openfold
directory/bin/python chimerax/site-packages/openfold/download_weights.py
openfold ligandtable runDirectory [ alignTo atomic-model ]
The openfold ligandtable command displays a table for batch ligand predictions. Normally this command is not needed because ChimeraX batch ligand predictions are tabulated in the directory where the OpenFold run is made in a comma-separated value file with suffix ".oflig" and you can open this file to show the table of results. But in cases where this file is missing you can recreate the table with this command which searches the prediction results extracting the scores, and searches the ".json" input file to find the SMILES strings for the ligands.
> cd C:\Users\username\openfold\Scripts > pip.exe uninstall torch > pip.exe install torch --index-url https://download.pytorch.org/whl/cu118
$ cd ~/openfold3/bin $ ./pip uninstall torch $ ./pip install torch --index-url https://download.pytorch.org/whl/cu118