

 about
projects
people
publications
resources
resources
visit us
visit us
search
search
about
projects
people
publications
resources
resources
visit us
visit us
search
search
Quick Links
Recent Citations
Cryo-EM structure of the vaccinia virus entry fusion complex reveals a multicomponent fusion machinery. Lin CS, Li CA et al. Sci Adv. 2026 Jan 16;12(3):eaec0254.
Structural insights into the activation mechanism of the human metabolite receptor HCAR1. Gao M, Zang S et al. Sci Signal. 2026 Jan 6;19(919):eadw1483.
Crystal structure of Methanococcus jannaschii dihydroorotase with substrate bound. Vitali J, Nix JC et al. Acta Crystallogr F Struct Biol Commun. 2026 Jan 1;82(Pt 1):23-31.
Correlation between solvation free energy and solute-solvent interaction energy in energy representation theory. Maruyama Y, Matubayasi N. J Phys Chem B. 2025 Dec 25;129(51):13230-13241.
Structural snapshots capture nucleotide release at the μ-opioid receptor. Khan S, Tyson AS et al. Nature. 2025 Dec 18;648(8094):755–763.
Previously featured citations...Chimera Search
Google™ SearchNews
December 25, 2025

|
September 22, 2025
Mac users may wish to defer upgrading to MacOS Tahoe. Currently on that OS the Chimera graphics window is shifted so that it covers the command and status lines.
March 6, 2025
Chimera production release 1.19 is now available, fixing the ability to fetch structures from the PDB (details...).
Previous news...Upcoming Events

UCSF Chimera is a program for the interactive visualization and analysis of molecular structures and related data, including density maps, trajectories, and sequence alignments. It is available free of charge for noncommercial use. Commercial users, please see Chimera commercial licensing.
We encourage Chimera users to try ChimeraX for much better performance with large structures, as well as other major advantages and completely new features in addition to nearly all the capabilities of Chimera (details...).
Chimera is no longer under active development. Chimera development was supported by a grant from the National Institutes of Health (P41-GM103311) that ended in 2018.
Feature Highlight
|
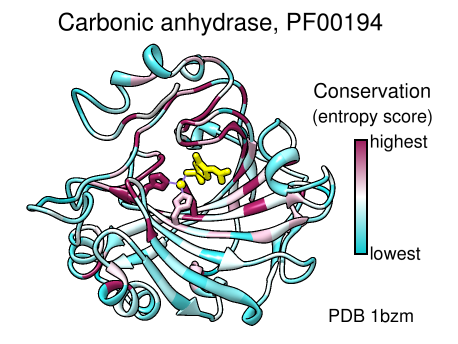
A structure can be colored to show attributes such as residue conservation. Opening a sequence alignment in Chimera shows it in Multalign Viewer and automatically associates sequences with structures as appropriate. Residues of alignment-associated structures are assigned conservation values; available measures include entropy, variability, and sum-of-pairs. The figure was created using the PFAM Carb_anhydrase seed alignment PF00194_seed.slx (see image) and includes 2D labels and a color key. See also: mapping sequence conservation
(More features...)
Gallery Sample
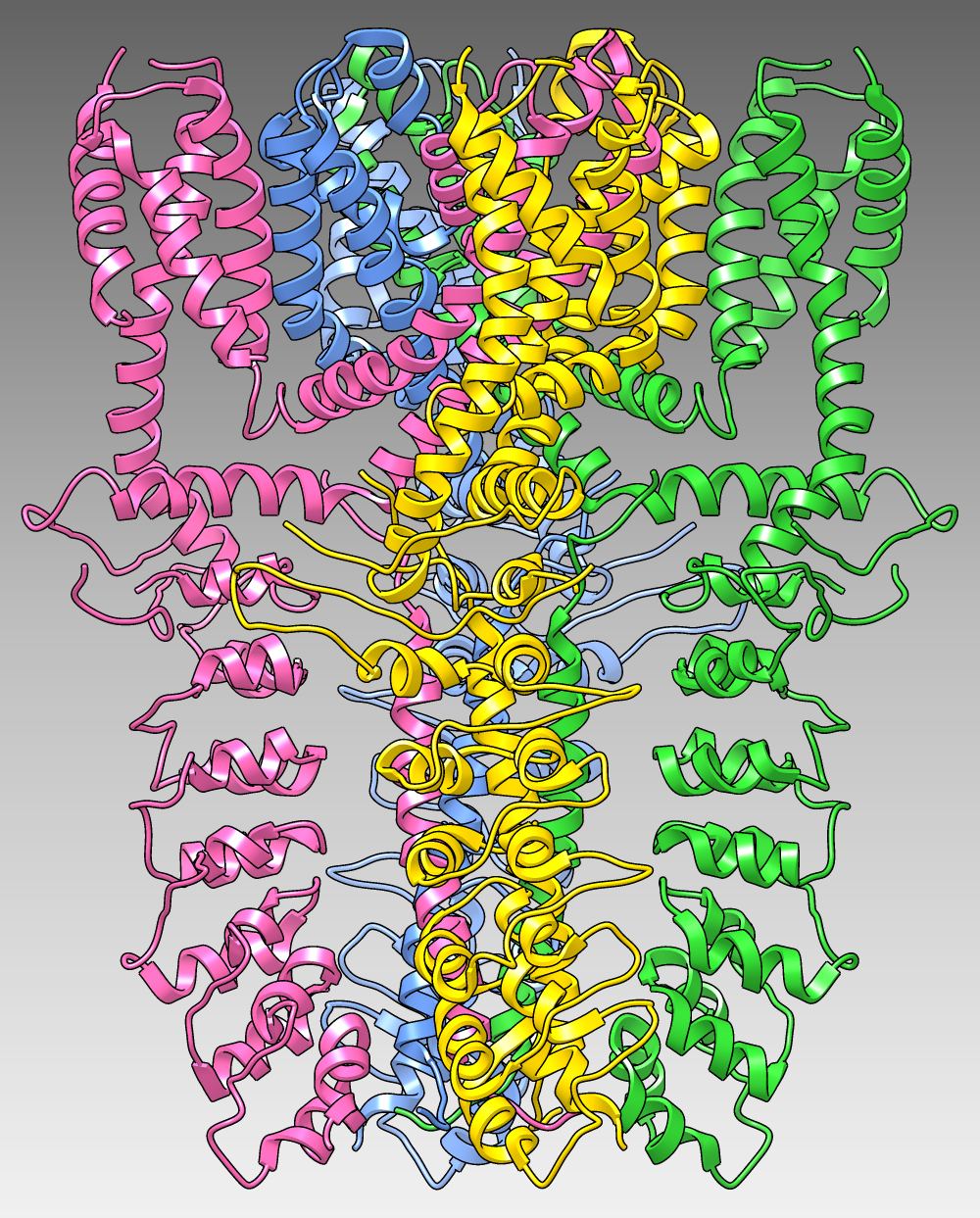
The image shows the structure of the human TRPA1 ion channel (wasabi receptor) determined by electron cryo-microscopy, Protein Data Bank entry 3j9p. The four subunits of the tetramer are shown as ribbons in different colors over a dark-to-light gradient background. (More samples...)
About RBVI | Projects | People | Publications | Resources | Visit Us
Copyright 2018 Regents of the University of California. All rights reserved.